Selecting the Right COVID-19 Code: Should You Use CPT or HCPCS?
Selecting the Right COVID-19 Code: Should You Use CPT or HCPCS?
Do you need to learn how to apply the COVID-19 codes for vaccines or telehealth services? Enroll in the latest COVID-19 training from YES HIM Education.
CPT Codes for COVID-19
On March 11, the World Health Organization (WHO) issued a declaration of the COVID-19 as a global pandemic. Two days later, the AMA announced the development of a specific code for laboratory testing for the coronavirus, code 87635 (AMA, 2020). The code is available for immediate use for laboratories, hospitals, and health care systems. UPDATE 4/10/20: The AMA fast-tracked the approval of two new CPT codes for COVID-19 antibody blood testing (AMA, 2020). The new codes were published April 10, 2020, and are effective immediately.
According to AMA President Patrice A. Harris, MD, MA, “Moving as quickly as possible to put in place a CPT code for a novel coronavirus test will bolster a data-driven response to the COVID-19 disease outbreak in the United States. By streamlining the flow of information on novel coronavirus testing, a new CPT code facilitates the reporting, measuring, analyzing, researching and benchmarking that is necessary to help guide the nation’s response to the public health emergency” (AMA, 2020).

How to Use CPT Code 87635
The AMA issued a special edition of the CPT Assistant available on its website. Included within the CPT Assistant articles is a fact sheet that describes the new code and provides a clinical example for use of the code and description of the testing procedure. Some questions and answers are also provided. Note that AMA made the code available for reporting on March 13. Individual payers will determine if the code can be used retroactively for testing performed previous to that date. The AMA will publish the code in the Pathology and Laboratory section of the 2021 edition of CPT. There are existing CPT codes that mention testing for coronavirus within their code description; however, the new code is used for testing for severe acute respiratory syndrome coronavirus 2 or COVID-19.
The AMA also released special coding advice for COVID-19. Included in this document are 11 scenarios for patient encounters, most involving either suspected or confirmed COVID-19 infection. The document also includes information on the Evaluation and Management coding for the cases, along with applicable ICD-10-CM diagnosis codes. The advice includes information on place of service and telehealth visits, as well as links to information about flexibility in the use of telemedicine afforded during the pandemic.
Additional COVID-19 Coding Updates
- Provisional Codes Now Available to Report Pfizer COVID-19 Vaccine for Young Children
- AMA Announces Administration Code for Third Dose of Pediatric Pfizer COVID-19 Vaccine
- CMS Announces New ICD-10 Codes for Vaccination and COVID-19 Treatments
- As More Americans Get COVID-19 Shots, Vaccine Codes are a Priority
- 2022 CPT Code Updates Feature 24 COVID-19 Vaccine-Related Codes
- AHIMA/AHA Update FAQ Documents for COVID-19 Coding
- 2022 ICD 10 CM Updates Now Available – Post COVID-19 Codes Included
- AMA Releases CPT Codes for Novavax COVID-19 Vaccine
- How to Code COVID-19 Treatment
- Updates to FAQs for COVID-19 Immunization Reactions
- COVID-19 Coding Update Course Reviews New ICD-10 Codes & Guidelines
- How to Code Post-COVID-19 Recovery Programs
- Third Group of CPT COVID-19 Vaccine Coding Guidelines from AMA
- 27 New ICD-10 Codes for COVID-19 Related Conditions
- Review These New CPT Codes for COVID-19 Vaccines for the AMA
- Update on Billing COVID-19 Vaccine
- New CPT Codes to Document COVID-19 & Respiratory Syncytial Virus Detection Test
- Latest CPT Codes 86413 & 99072 Report COVID-19 Special Services & Tests
- Four New CPT Codes for Lab Tests Available Now
- Twelve New ICD-10-PCS Hospital Procedure Codes Released for COVID-19 Treatment
- COVID-19 Antigen Testing Code 87426 Available for Immediate Use from AMA
- AMA Releases Two New CPT Codes for COVID-19 Antibody Detection
HCPCS Codes for COVID-19 Testing
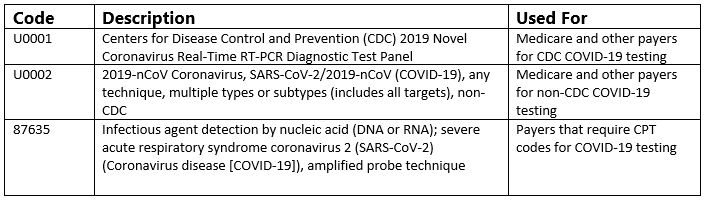
The Centers for Medicare and Medicaid Services (CMS) released two new HCPCS codes for coronavirus testing for Medicare claims. Code U0001 for CDC testing was first approved, followed by code U0002 for non-CDC testing (CMS, 2020). These codes will allow those laboratories conducting the tests to report the specific test instead of an unspecified code, allowing for better tracking of testing for COVID-19. The HCPCS codes are accepted as of April 1, 2020, for dates of service of February 4, 2020, or after. Review our previous article for more information.
CDC Updates on Testing Guidance
Initial guidance from the CDC recommended collection of multiple specimens for testing; an upper respiratory nasopharyngeal (NP) swab and an oropharyngeal (OP) swab. The CDC updated its interim guidance for COVID-19 testing on 3/15/2020 to recommend collecting only an NP swab and noted that the collection of an OP specimen is of lower priority. However, if both samples are collected, they should be combined in the same tube.
The CPT Assistant article included a question on reporting more than one specimen (in the case that both NP and OP swabs are collected). The authors responded that for this scenario, coders report the 87635 code twice with the 59 modifier appended to the second code. If only an NP swab is collected as recommended by the updated CDC guidelines, the 87635 code is reported once.

Which COVID-19 Code To Use? Should Coders Submit More Than One Code?
The reporting for COVID-19 testing is dependent on the payer. Coders should use either an HCPCS or CPT code; there should not be more than one COVID-19 testing code on a given claim. Coders use the HCPCS codes for Medicare claims, and other payers may require these codes. For example, some plans call for the HCPCS code U0002. Other payers that require CPT codes will use the 87635 code. Contact your payer directly for individualized guidance.
Diagnosis Coding Information
As outlined in our other article, a new ICD-10-CM diagnosis code is available for use on April 1, 2020. You can find information on this code and other guidance here. AHIMA and AHA also recently published a series of questions and answers for coding of COVID-19-related encounters.
Updates Continue
As researchers discover more about COVID-19 diagnosis, treatment, and prevention, agencies and organizations will develop additional codes and guidance. We will continue to report on these developments. Check out the COVID-19 Resource Center for the latest updates, news, and coding advice.
In addition, do you need to learn how to code the COVID-19 vaccines or telehealth services? Enroll in the latest COVID-19 training from YES HIM Education.


